Diamond (Guide)
The Diamond

The cubic modification of the chemical element carbon is called diamond. So much for the relatively sober depiction of a material that has fascinated people for thousands of years. Whether in the crown jewels of the English royal house or the countless mythical legends and stories, diamonds have become an integral part of human history.
In the following paragraphs we would like to give a brief insight into the exciting field of gemmology. For this purpose, we will first deal with the etymological and historical background of diamonds. After that, we will go into the natural and synthetic creation process of diamonds in order to explain their value and the closely related 4 C's (Cut, Clarity, Colour, Carat) in more detail. In addition, the most popular cuts for diamond jewellery are introduced and an overview of the most valuable and famous diamonds in the world is given.
Finally, we critically examine the methodology of diamond determination and give valuable tips and tricks.
Etymology and historical background
The word diamond is derived from the late Latin term "diamantem" or "diamas", which is a linguistic adaptation of the ancient Greek "adámas" for " invincible". To describe the diamond as "the invincible" still makes sense today, since in the Mohs scale of hardness this mineral, with a value of 10, is the hardest known natural substance.
The oldest recorded diamond findings can be located in India and date back to the year 4000 BC. However, diamonds were neither worked nor used as tools there, as it was believed that they would lose their magical effect. The fact that diamonds can be worked and therefore also cut was first discovered in the 13th century AD. The almost typical brilliant cut of diamonds that is used today was not even developed until 1910.
Natural origin
Diamonds are usually only formed in the Earth's mantle and only between depths of 150 and 660 kilometres. Only here exists the right pressure-heat combination in association with sufficient carbon (graphite) deposits that diamonds can be formed naturally.
Since mining at such depths is almost impossible, it is necessary to rely on transporting the stones for industrial extraction by natural means. Volcanoes usually bring diamonds to or near the earth's surface through eruptions. Only through these natural processes is the industrial mining of diamonds possible at all. Comet impacts can also lead to the formation of diamonds. However, the diamonds found in and around the craters are far too small for gemstone processing.
Synthetic production
In 1953, the physicist Erik Lundblad succeeded in synthetically producing a diamond for the first time. With the help of the high-pressure, high-temperature process, it has been possible to produce diamonds artificially since 1955. The basic material graphite is compressed with the addition of a catalyst for acceleration, with the help of a hydraulic press under such high pressure (60,000 bar) that graphite is transformed into diamond by pressure and heat. Despite the addition of catalysts, the process of transformation still takes several weeks.
Diamonds can also be produced by detonation synthesis or shock wave synthesis. Both processes use explosives to generate the necessary pressure and heat for the conversion process.
For some years now, there has also been great progress in the field of lab-grown diamonds. With these diamonds, a new era has begun for the diamond jewellery sector: They show the same fire, the same hardness as well as the same inimitable brilliance. They consist of 100 % carbon and have the same chemical composition as a mined diamond. Lab-grown diamonds not only offer a price advantage over naturally mined diamonds, but are more environmentally friendly in the way they are produced and are always conflict-free. Such diamonds are a responsible choice as they do not require diamond mining.
The 4 Cs
The diamonds get their value as a gemstone due to their extraordinary light refraction properties, enormous shine and striking dispersion. These properties are best utilised in the brilliant cut to achieve maximum light reflection and create the often quoted "fire" in the eye of the beholder.
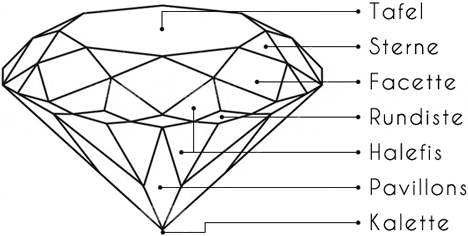
The standard number of facets in a brilliant cut is 57 (58 if the lower tip of the diamond is counted as a surface).
An ideal cut directs the light via the table (upper surface) into the interior of the diamond. The lower lateral halefis or calette edges reflect the light rays to the opposite side and thus prevent the light wave from escaping. The light wave is emitted via the upper facets of the brilliant-cut diamond, which produce the typical sparkle.
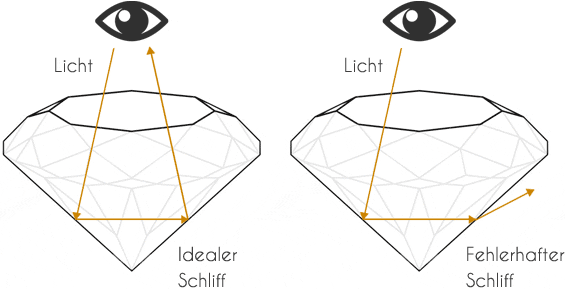
However, a diamondaire always faces the same dilemma with every rough diamond. On the one hand, the profitability of the diamond increases with the quality of the cut, but at the same time it decreases due to the inevitable loss of weight. The weight loss of a rough diamond to an "ideal cut" can be up to 70%. Four characteristics determine the value of a diamond, also known as the 4 Cs. (Cut, Clarity, Carat, Colour).
In the following paragraph, the value-determining characteristics of a diamond are briefly discussed.
Cut
Only the cut transforms raw crystals into sparkling gemstones. For centuries, the techniques for refining this rare material have been further developed to ignite the fire in diamonds.
With faceted diamonds, the so-called quality class of the cut is divided into five stages.
-
Quality grades of the cut
- Ideal cut (optimum proportion ratios ensure maximum brilliance)
- Very good (Excellent proportions and brilliance, hardly any external features)
- Good (few external characteristics, good brilliance but proportions with slight deviations)
- Medium (reduced brilliance, increased external characteristics and significant proportional variation)
- Low (strongly reduced brilliance, clear proportional deviations as well as numerous and/or large deviations)
Inclusions and other optical properties of rough diamonds have led to the development of various types of cuts. Brilliance, lustre and colouring are not the only value-determining properties of diamonds, but also their weight, indicated in carats. A diamond cutter has not only the task to cut a diamond in the highest quality class, but also to decide for a type of cut, which is as high as possible in the total weight of the stone. results.
The following part b) gives a brief overview of the possible types of cuts.
-
Types of cut of the diamond
marquise cut
brilliant cut
cylindrical grinding
trillian cut
oval cut
drop shape
carré cut
heart cut
emerald cut
baguette cut
princess cut
antique cushion cut
octagon cut
briolette cut
trapezoidal grinding
hexagon cut
Carat

As already briefly mentioned before, the so-called carat indicates the mass, i.e. the weight of gemstones. A metric carat is 0.2g.
The weight of the carat has its origin in the dried seed of the carob tree, since it was previously considered to be very constant in weight and size and could therefore serve as a reference.
Today, the weight of a diamond is measured to the nearest hundredth. A carat is therefore divided into 100 points, which are assigned to the corresponding exact unit of weight. A diamond with 50 points weighs exactly half a carat. Unfortunately, the carat does not have a legal unit symbol, so there are several abbreviations in circulation. It often happens that the two units carat (gold alloy) and carat (gemstone weight) are confused. Carat is a unit of measurement of gemstones and 1 carat is equal to 0.2 grams. Consequently, the heavier and larger the gemstone, the more valuable it is. A proud five-carat diamond weighs 1 gram. That may not be much, but such a diamond is very valuable. The common abbreviation for carat is ct. The other form indicates the purity of gold. Jewellery made of pure gold with nothing added is called 24-carat gold jewellery. 24-carat gold consists at least theoretically of 99.9% pure gold. More common in jewellery making then are 14 carat and 18 carat gold. These pieces of gold jewellery are not only made of pure gold, but also contain other precious metals.
Colour
The colouring of the rare mineral also has an influence on its value. The so-called "colour designation" of the diamond begins with the letter D. A diamond with this colour designation is absolutely colourless and represents the best possible degree. The colour designations come from the GIA (Gemological Institute of America), a research institute founded in 1931. The colour scale ranges from D (ultra-fine white) to Z (maximum tinted yellow). The following table gives an overview about the shades still referred to by the GIA as white (1-11).
| Nat. name | Int. name | GIA designation | Occurrence | Hue | Colour scale | |
|---|---|---|---|---|---|---|
| 1. | Superfine White+ | River | D | very rare | colourless | |
| 2. | Superfine White | River | E | very rare | colourless | |
| 3. | Fine White+ | Top Wesselton | F | very rare | colourless | |
| 4. | Fine White | Top Wesselton | G | rare | almost colorless | |
| 5. | White | Wesselton | H | rare | almost colorless | |
| 6. | Lightly tinted white+ | Top Crystal | I | rare | almost colorless | |
| 7. | Lightly tinted white | Top Crystal | J | rare | almost colorless | |
| 8. | Tinted White+ | Crystal | K | rare | tinted white | |
| 9. | Tinted White | Crystal | L | rare | tinted white | |
| 10. | Tinted 1 | Top Cape | M, N | abundantly available | tinted | |
| 11. | Tinted 2 | Cape | O | abundantly available | tinted |
Clarity
The degree of "clarity" of a diamond is significantly influenced by the inclusions or impurities in the stone. A diamond is designated "fl" (flawless), i.e. "absolutely" pure, if an expert's eyes cannot detect any inclusions even at 10x magnification. The following table gives a brief overview of the different clarity classifications of the diamond..
| Abbreviated designation | Meaning | Description |
|---|---|---|
| fl | flawless | Flawless even at 10x magnification (no inclusions or external defects visible) |
| if | internally flawless | Except for possible surface traces from the processing of flawless interior |
| vvs1/vvsi | very, very small inclusions | Even at tenfold magnification, inclusions are very, very difficult to detect. |
| vvs2 | very, very small inclusions | Even at tenfold magnification, inclusions are very difficult to detect. |
| vs1/vsi | very small inclusions | Inclusions are difficult to detect at ten times magnification. |
| vs2 | very small inclusions | Inclusions are visible at tenfold magnification. |
| si1 | small inclusions | Inclusions are easily recognized at tenfold magnification. |
| si2 | small inclusions | Inclusions are very easy to see at tenfold magnification, but not with the naked eye. |
| pi1 | Piqué I | Inclusions are very difficult to see with the naked eye, but do not reduce the brilliance. |
| pi2 | Piqué II | Inclusions visible to the naked eye, only weakly reduce brilliance |
| pi3 | Piqué III | Inclusions are easily visible to the naked eye and significantly reduce brilliance. |
The most precious diamonds in the world
| Name | Note | Carat |
|---|---|---|
| Cullinan | Biggest diamond ever found. Split into a total of 105 stones, the largest 9 pieces form an elementary part of the British crown jewels. | 3016.7 |
| Excelsior | Found in South Africa, it is the second largest diamond ever found and was split into a total of 22 stones. The largest of these weighs a proud 373.75 carats and is owned by Robert Mouawad. | 995.2 |
| Star of Sierra Leone | Was discovered in 1972 in Sierra Leone. With a gross weight of 193.78 grams, it is the third largest diamond ever found in the world. | 968.9 |
| Golden Jubilee | It's the largest cut diamond in the world. In 1985 he was promoted in South Africa and after one year of work he retained an incredible weight of 545.67 carats. In 1995 he received the blessing of Pope John Paul II and is is now part of Thailand's crown jewels. | 755 |
| Orloff | Sitting in the golden sceptre of the Russian tsar and today can be seen in the Diamond Fund Exposition in the Kremlin, Moscow. According to legend, it was stolen from the eye of the Indian statue of Brahama the god who cursed it. that he had been. | 189.62 |
| Koh-i-Noor | It is not known when the Koh-i-Noor was found. However, his place of discovery was most probably in India. Today he lies in the Tower of London. | 186 |
| Hope Diamond | His discovery year is also unknown. The brilliant blue diamond first appeared in history in 1642. He is also said to have been in the possession of the self-proclaimed Sun King Louis XVI. | 45.52 |
| Green Dresden | The largest naturally green cut diamond. Its bright green color results from the naturally occurring radioactivity of its deposit. Today he can visit the "New Green Vault" in the Residenzschloss Dresden. | 41 |
Identification of real stones
It always happens that gemstone jewellery from an old family estate is found. This immediately raises the question: Are the stones genuine? Is it possible to determine this from home under the living room lamp?
All too often in times of economic crises and high inflation rates, people talk about diamonds as a "safe" investment. But how do I know if I'm getting a real diamond and if the declared amount also corresponds to the true value?
We would like to point out at this point that it is very difficult, if not impossible, to determine a diamond as "genuine", i.e. as a natural product created over millions of years. This is due to the centuries-long efforts and ultimately also the success of science to produce diamonds artificially. We always advise you to consult a trusted jeweller for a determination of authenticity.
In the next paragraph, a small overview of the possible determination methods is given and their risks and grey areas are explained.
There are ways and methods how to distinguish a real diamond from the probably best known imitation diamond, the zirconia, even from home.
Density measurement
A real diamond is significantly lighter than a zirconia with the same size. This is due to the lower density of the diamond. A genuine one-carat brilliant-cut diamond has a diameter of 6.5 mm. A zirconia of the same cut and weight has a diameter of only 5.4mm. Of course, this principle also works the other way round. If you have two stones with the same diameter, the diamond will always be significantly lighter than the zirconia.
The problem with this method, however, is that a certified genuine diamond of the same weight as the specimen to be tested must first be available in order to produce comparative values. There is also the problem of the cut quality. It can significantly influence the diameter of the stone with the same carat number.
The Glass Test
According to popular belief, distinguishing a cubic zirconia from a diamond can perform a glass-cutting test. A diamond cuts glass, a zirconia not necessarily. However, this is clearly not the case. High-quality zircons are quite capable of cutting through glass, which makes this test only conditionally suitable for checking whether it is a zirconia or a real diamond.
The breaking test
Under no circumstances should the stone be struck with a hard object in order to verify its authenticity. Although the diamond is the hardest natural substance we know, it is relatively easy to split. This is due to the molecular structure of the diamond. Parallel lattice bonds define the fracture edges.
The condensation test
When breathing on glass panes, the moisture stored in our breath lingers and condenses. This also happens with real diamonds, but the condensation moisture only lasts for a fraction of the time. If a breathed-on gemstone is clearly fogged up, it can be assumed that it is not a real diamond.
The water drop test
If a drop of water is added to the tablet of the stone, a high, arched dome forms in real diamonds. A zirconia cannot do this. A drop of water melts on its board.
The luminous calculability
Take a white sheet of paper and draw a straight line on it with a pencil. Now take your gemstone and place it on the line with the tablet facing down. If you see the line (broken if necessary) it is a zirconia. Real diamonds have such a high refractive index that you cannot see through them.
thermal conduction
The atomic structure of diamonds ensures extraordinarily good heat conduction. As a result, real diamonds always feel cold because they absorb the heat of our skin immediately. Zirconia have extremely poor heat conducting properties.
Distinguishing diamonds from other stones
Moissanite

Probably the most difficult "diamond doppelganger" to recognise is moissanite. Even experts have a hard time distinguishing moissanite from real diamonds. A diamond tester will always indicate a genuine diamond, although a moissanite is being tested. This is due to the almost identical thermal conductivity of the two stones. A moissanite also passes the density test described above because the mass behaviour is very similar. It is also almost as hard as a real diamond and can reach a value of 9.25 on the Mohs hardness scale. Moissanite and diamonds also show the same results in the condensation test and water drop test. Special moissantite testing devices are constantly being improved. But even they cannot deliver a really reliable result, because the manufacturing processes for moissanite are also constantly improving. Only the light refraction index is able to unmask the moissanite, as it has birefringent light refraction properties. This ultimately leads to facet doubling and the moissanite even sparkles a little more than the real diamond. However, it requires a very strong microscope and the trained eye of an expert to be able to determine the effect of facet doubling. With small stones of less than 2 mm or even with much larger but set stones, a distinction is not possible beyond doubt.
Loose, i.e. set stones, can be safely tested in diiodomethane without any doubt. Since diamonds have a density of 3.52, moissanite a density of 3.21 and diiodomethane a density of 3.32, the diamond sinks. The moissantite, on the other hand, floats on the surface.
Zircon
A real diamond does not break up the light into its individual spectral colours but reflects it almost unbroken. The sparkle of a real diamond is therefore colourless. A zircon, on the other hand, reflects a wide spectrum of colours, but mostly a slightly orange-reddish glow.
White sapphires
Contrary to the misconception that sapphires are always blue, sapphires actually occur in almost every colour. With a Moh's hardness of 9, they are somewhat softer and thus have less mass than diamonds. White or colourless sapphires do not have the light-dark contrast typical of real diamonds. If the inner colour play between light and dark shows flowing rather than sharp transitions, it is probably a white sapphire.
The white topaz
In contrast to diamonds, white topaz is much softer. A diamond is one of the hardest materials known to mankind. So it is quite unlikely to see scratches on a diamond. If scratches should be recognizable, it is a matter of is unlikely to be a real diamond, but a topaz.
Certificates
Especially when diamonds are considered as investments, they should never be purchased without a certificate. Trustworthy certificates of authenticity that provide information about the DNA of a diamond are only issued by the GIA (Gemological Institute of America), the AGS (American Gem Society) of EGL (European Gemological Labratory) and the IGI (International Gemological Institute).
For more detailed information about the individual institutes, we have compiled the respective web links for you here.
- GIA
- http://www.gia.edu
- AGS
- http://www.americangemsociety.org
- EGL
- http://www.eglinternational.org
- IGI
- http://www.igiworldwide.com
If for any reason no certificate is available, we expressly advise against a purchase. Only a verifiable find before 1953, i.e. the date of manufacture of the first successfully synthetically produced diamond, then offers a Security.
Sources
- http://de.wikipedia.org/wiki/Schliff_%28Schmuckstein%29
- http://www.leifiphysik.de/themenbereiche/lichtbrechung/ausblick
- http://www.padi1990.homepage.eu/infos_ueber_diamanten_03985675.html
- http://de.wikihow.com/Woran-man-erkennt-ob-ein-Diamant-echt-ist
- http://de.wikipedia.org/wiki/Diiodmethan
- http://de.wikipedia.org/wiki/Moissanit
- http://suite101.de/article/diamant-und-brillant--echtheit-und-qualitaet-selbst-erkennen-a81719#.U_xq0mOh5OI
- http://www.indimant.biz/fileadmin/bilder/PDFs/4.1_diamant_u_s_eigenschaften.pdf
- http://de.wikipedia.org/wiki/Gr%C3%BCnes_Gew%C3%B6lbe#Neues_Gr.C3.BCne_Gew.C3.B6lbe
- http://de.wikipedia.org/wiki/Hope-Diamant
- http://de.wikipedia.org/wiki/Orlow-Diamant
- http://de.wikipedia.org/wiki/Excelsior_%28Diamant%29
- http://de.wikipedia.org/wiki/Golden_Jubilee
- http://de.wikipedia.org/wiki/Star_of_Sierra_Leone
Image Sources
- Main image diamond: iStock.com/Shenki
- Cut types of the diamond: Leysan/stock.adobe.com
- Johannisbrot cavern: emuck/stock.adobe.com
- Comparison of light refraction moissanite and diamond: http://cdn.moissanite.com/media/wysiwyg/cms/educate/jfire_faq.jpg
¹Free shipping from 100 € shopping basket value for deliveries within Germany.
²Offer ends midnight 02/03/2025. No minimum order value. Only applies to our name jewellery designer. No combination with other discounts or vouchers. Only redeemable once per customer. For private customers only.















